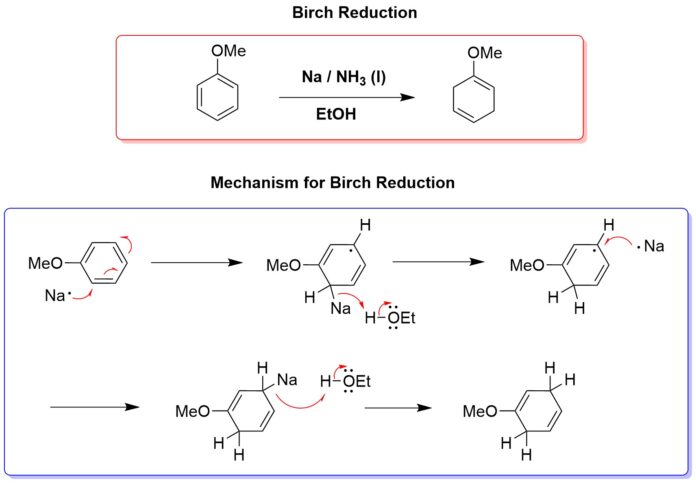
🌿 Birch Reduction – Overview
The Birch reduction is a partial reduction of aromatic rings using:
- Sodium (Na) or lithium (Li) metal
- In liquid ammonia (NH₃)
- With an alcohol (ROH) as a proton source
It converts benzene or substituted aromatics into 1,4-cyclohexadienes.
⚗️ Mechanism Steps
- Electron transfer (1st reduction):
Sodium (or lithium) donates an electron to the aromatic ring → forms a radical anion. - Protonation:
The radical anion is protonated at the position of highest electron density by the alcohol. - Second electron transfer:
Another electron is added to form a carbanion intermediate. - Final protonation:
The carbanion is protonated again → gives the 1,4-cyclohexadiene product.
💡 Effect of Electron-Donating Groups (EDGs)
Examples of EDGs: –OCH₃, –OH, –NH₂, –CH₃
- EDGs donate electron density into the aromatic ring via resonance or induction.
- This increases electron density at the ortho and para positions.
- During reduction, the negative charge of the intermediate (radical anion/carbanion) avoids EDG-substituted carbons, because both are electron-rich.
- Therefore, protonation occurs at positions meta to the EDG.
🔍 Result
- Aromatic rings with electron-donating substituents give 1,4-cyclohexadienes where the double bonds are located near the EDG, and protons are added at meta positions.
- In contrast, electron-withdrawing groups (EWGs) lead to protonation ortho/para to the substituent.
🧪 Example
Anisole (methoxybenzene) + Na/NH₃/EtOH →
→ 1-methoxy-1,4-cyclohexadiene,
where reduction occurs at C-3 and C-5 (meta to –OCH₃).