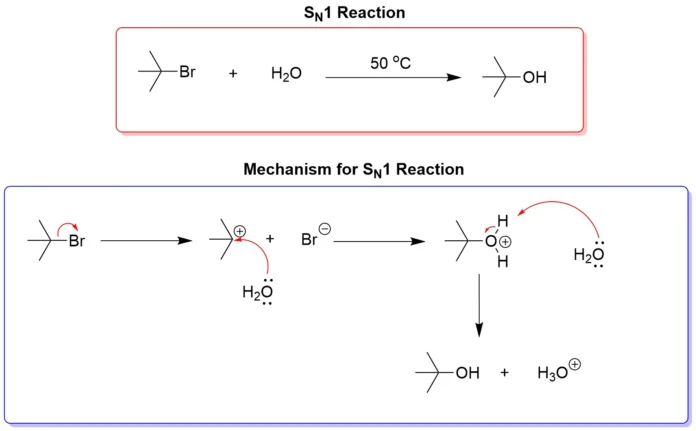
SN1 reactions are substitution reactions where the rate-determining step involves only one molecular entity, making the reaction unimolecular.
Mechanism:
- Formation of Carbocation (Rate-Determining Step):
- The leaving group (e.g., a halide) departs from the substrate, forming a carbocation.
- This step is slow and determines the reaction rate.
- Nucleophilic Attack:
- A nucleophile attacks the planar carbocation intermediate.
- This can lead to either retention or inversion of configuration at the reaction center, often resulting in a racemic mixture if the substrate is chiral.
Key Features:
- Rate Law: Rate = k[substrate]k[\text{substrate}]k[substrate]
(Rate depends only on the concentration of the substrate, not the nucleophile.) - Carbocation Stability: The reaction favors substrates that can form stable carbocations (e.g., tertiary > secondary > primary).
- Polar Protic Solvents: These solvents stabilize the carbocation and leaving group, enhancing the reaction rate.